PSE-Lab just published a new paper!
The joint efforts of medical doctors and engineers took to the publication of a new paper on “Clinical Endocrinology” of Wiley:
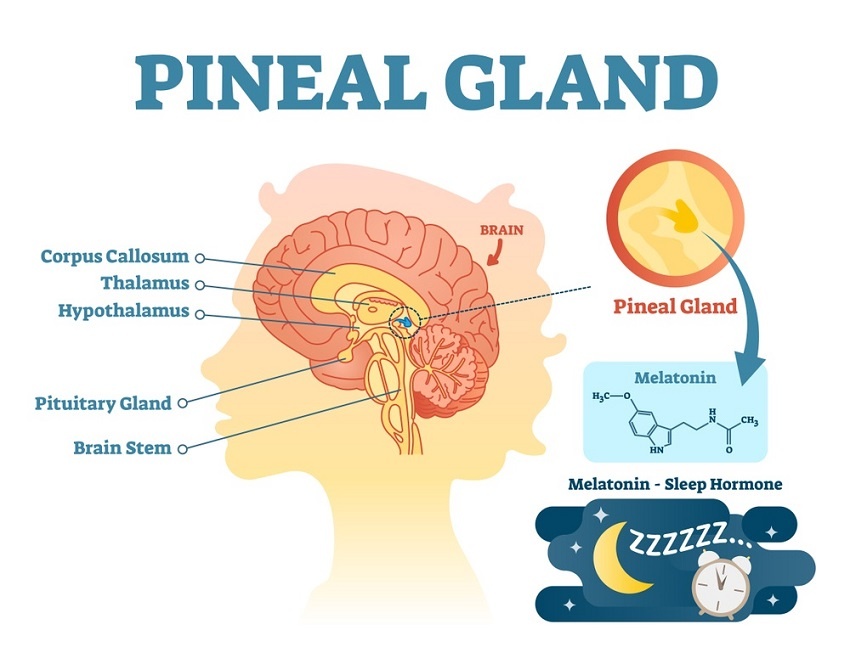
Different routes and formulations of melatonin in critically ill patients. A pharmacokinetic randomized study
Giovanni Mistraletti, Rita Paroni, Michele Umbrello, Bedrana Moro Salihovic, Silvia Coppola, Sara Froio, Elena Finati, Paolo Gasco, Adriana Savoca, Davide Manca, Davide Chiumello, Russel J. Reiter, Gaetano Iapichino
DOI:Â https://doi.org/10.1111/cen.13993
Abstract
Background and objectives: Critically ill patients present reduced endogenous melatonin blood levels, and they might benefit from its exogenous supplementation. The aim of this research was to evaluate the feasibility of different routes of administration and drug formulations of melatonin. The efficiency of absorption was assessed as well as the adequacy in achieving and maintaining the physiological nocturnal blood peak.
Methods: Twentyâ€one highâ€risk critically ill patients were randomly assigned to receive melatonin either: (a) per os, as a standard tablet (STâ€OS), (b) per os, as a suspension in solid lipid nanoparticles (SLNâ€OS) or c) transdermal (TD), by applying a jellified melatonin microemulsion (μE) on the skin (μEâ€TD). SLNâ€OS and μEâ€TD were lipidâ€based colloidal systems. The endogenous melatonin blood values were observed for 24 hours; subsequently, melatonin 3 mg was administered and pharmacokinetics was studied for 24 hours further.
Results: In both groups that received STâ€OS and SLNâ€OS, the median timeâ€toâ€peak blood concentration was 0.5 hours; however, the area under the curve (AUC) after administration of SLNâ€OS was significantly higher than after STâ€OS (157386 [65732â€193653]vs 44441 [22319â€90705] pg/mL*hours,P = 0.048).μEâ€TD presented a delayed timeâ€toâ€peak blood concentration (4 hours), a lower bioavailability (AUC: 3142 [1344â€14573] pg/mL*hours) and reached pharmacological peak concentration (388 [132â€1583] pg/mL).
Conclusions: SLNâ€melatonin enterally administered offers favourable pharmacokinetics in critically ill patients, with higher bioavailability with respect to the standard formulation;μEâ€TD provided effective pharmacological blood levels, with a timeâ€conâ€centration profile more similar to the physiological melatonin pattern.
Keywords:Â critically ill patients, lipid nanovector encapsulation, melatonin, microemulsion, transdermal absorption