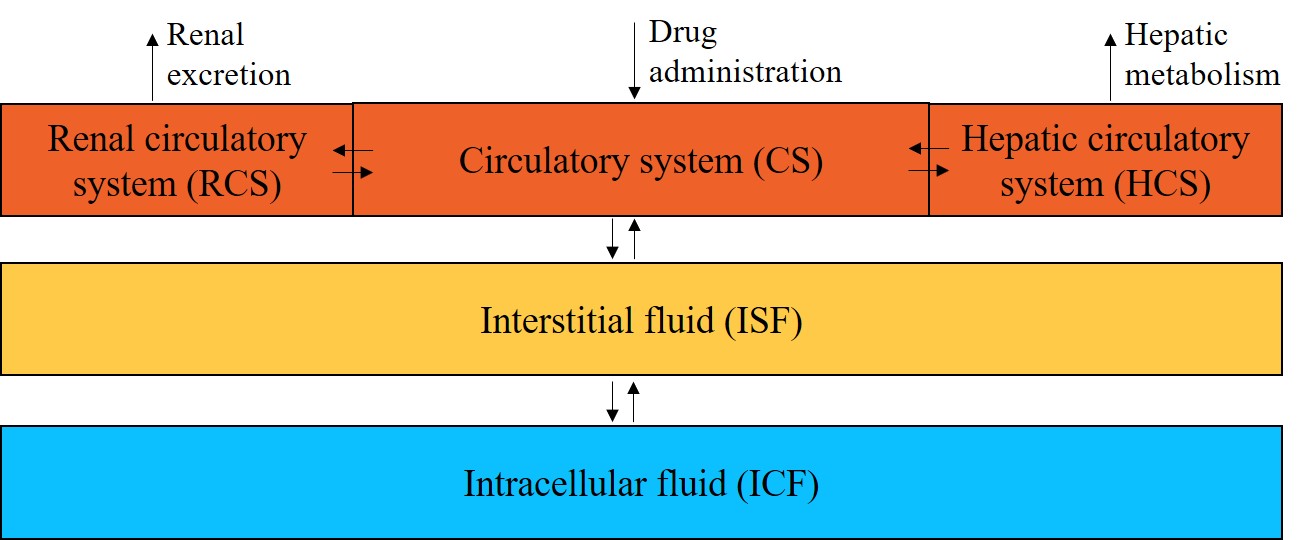
The PSE-Lab just published a new paper in a joint collaboration with IRCCS San Raffaele Scientific Institute, Milan. The paper presents a minimal physiologically-based pharmacokinetic (minimal PBPK) model for the intravenous administration of high-dose methotrexate, an anti-cancer drug, as the basis for the development of clinical tools able to suggest model-informed dosages and lead to improved treatment outcomes.
It is published as Open Access in “Cancer Chemotherapy and Pharmacology“, a Springer journal:
A minimal physiologically based pharmacokinetic model for high‑dose
methotrexate
Giuseppe Pesenti, Marco Foppoli, Davide Manca
DOI: 10.1007/s00280-021-04305-2
Abstract
Purpose High-dose methotrexate (HDMTX) is administered for the treatment of a variety of malignant tumors. Wide intra- and inter-individual variabilities characterize the pharmacokinetics of MTX, which is mostly excreted renally. HDMTX dosages are prescribed as a function of body surface area whereas dose adjustments depending on renal function are not well defined. We develop a population pharmacokinetic model with a physiological description of renal excretion as the basis for clinical tools able to suggest model-informed dosages and support therapeutic monitoring.
Methods This article presents a minimal physiologically based pharmacokinetic (PBPK) model for HDMTX, which specifically accounts for individual characteristics such as body weight, height, gender, age, hematocrit, and serum creatinine to provide individualized predictions. The model supplies a detailed and mechanistic description of capillary and cellular exchanges between plasma, interstitial fluid, and intracellular fluid compartments, and focuses on an individualized description of renal excretion.
Results The minimal PBPK model is identified and validated with a literature dataset based on Chinese patients suffering from primary central nervous system lymphoma. A comparison with a pharmacokinetic model from the literature suggests that the proposed model provides improved predictions. Remarkably, the model does not present any significant bias in a wide range of degrees of renal function.
Conclusion Results show that model predictions can capture the wide intra- and inter-individual variability of HDMTX, and highlight the role played by the individual degree of renal function. The proposed model can be the basis for the development of clinical decision-support systems for individualized dosages and therapeutic monitoring.
Keywords: Methotrexate · HDMTX · Pharmacokinetics · Minimal PBPK · Renal function
The full article can be freely viewed and downloaded here.